Launch Time: 2020-08-27 Views: 5972 Rely: 0 Started by: vincentwang
What’s PMTA?
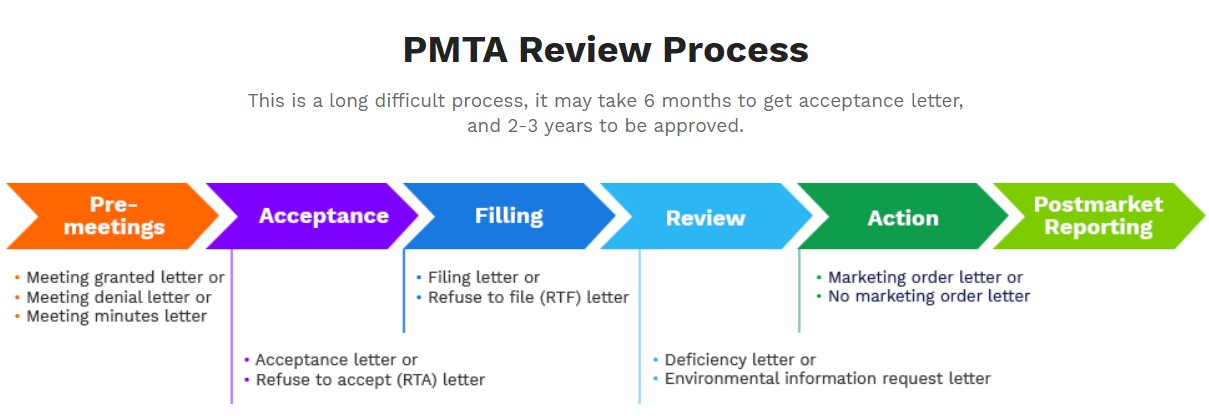
A PMTA is a Premarket Tobacco Product Application that all e-cigarette manufacturers have to submit to the FDA in order to continue marketing and selling their vaping products. PMTA requires the applicant to provide scientific data that demonstrates the product is appropriate for the protection of public health. (See, FDA’s website)
On May 10, 2016, the US FDA finalized its "deeming" rule, subjecting additional products to scrutiny under the Federal Food, Drug and Cosmetic Act as amended by the Family Smoking Prevention and Tobacco Control Act. Although the entire application is a long process, the FDA does not leave much time for the preparation of application materials. After several times of modifications on timelines, it finalizes the deadline for submission on Sep 9th, 2020.
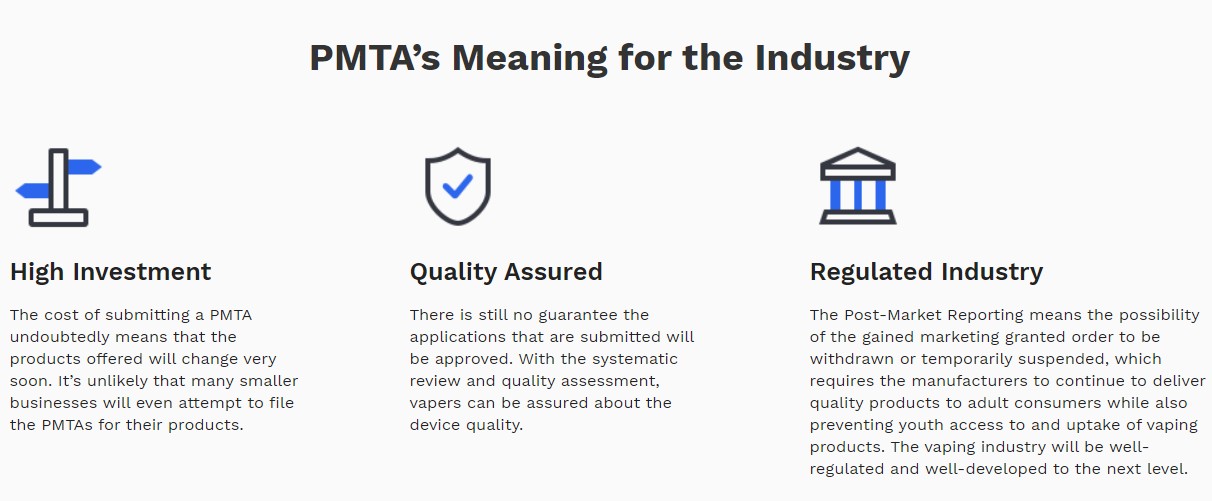
PMTA serves as the foundation of establishing long-term business locally due to its extremely high standards and complicated process. Besides the requirements the products must meet, a considerable investment of manpower, capital, and time are also necessary to a PMTA.